How Do Boats Corrode? Part 2: The Galvanic series and galvanic corrosion
Resources
For further reading on galvanic corrosion, check out these resources.
https://www.corrosionclinic.com/types_of_corrosion/Electrolysis-and-electrolytic-corrosion.htm
https://bestmarinesurveyor.com/galvanic-corrosion-can-happen-above-the-water-too/
https://abycinc.org/surveys/?id=1439795
Galvanic couples and the galvanic series
As we discussed in the article above, any time dissimilar metals are immersed in an electrolyte and electrons are allowed to freely flow between them (by the dissimilar metals making direct contact or being wired together), we create a galvanic couple. Galvanic couples can be a good thing (as we’ve discussed above) - such as in the case of a battery, or cathodic protection, and they can also be a bad thing - causing propellers and/or propeller shafts to corrode away. This type of corrosion is sometimes, erroneously, called “electrolysis”; but the correct term for it is galvanic corrosion. In this case, the less noble metal protects a less noble metal, but the less noble metal is an important piece of gear such as a propeller!
Because you now understand the fundamentals of how cathodic protection works, you already understand how these harmful cases of galvanic couples or galvanic corrosion work. Cathodic protection (desirable corrosion) and galvanic couples/galvanic corrosion operates on exactly the same principles. Let’s go into more detail about how different metals interact with each other when coupled together.
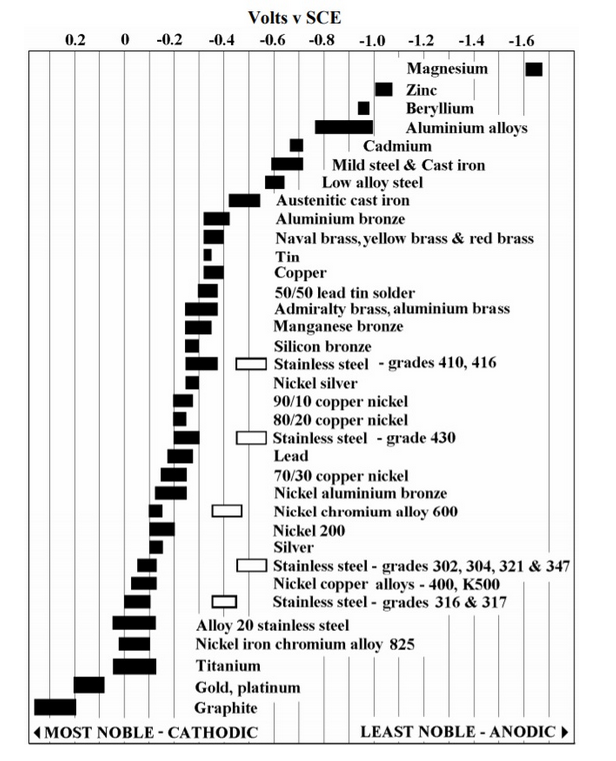
The following reference chart shows the potentials (compared to an SCE - or standard reference electrode) of metals in seawater. This chart can not only be used predict how susceptible to corrosion a metal is when submerged in seawater by itself, but can also be used to understand what metals serve as a sacrificial cathode (whether desired or not) when metals are combined and submerged.
To determine the compatibility of two metals in a galvanic couple, lookup the reference voltage (Volts v SCE in the chart) of each metal. Subtract the reference voltage of the least noble - anodic metal in the chart from the reference voltage of the most noble metal cathodic metal. In a harsh, saltwater marine environment, a difference of +/-0.2 V is generally considered to be enough of a voltage differential for corrosion to occur. By attaching sacrificial anodes that are less noble than all of the metals we want to protect to the propeller and prop shaft - “zincs” - made of magnesium (freshwater only), aluminum (salt and brackish water), or zinc (salt water) we introduce a third metal to the mix that will sacrifice itself and protect both metals.
Example: Many sailboats are built with a 316 stainless (or possibly even a more noble duplex stainless steel) prop shaft and a silicone bronze propeller. The voltage of 316 stainless, from the chart, is -0.05V. The voltage of silicone bronze, from the chart, is -0.28V. Calculating the voltage difference between the two metals: -0.28V - (-0.05V) = -0.23V; just over the threshold for galvanic corrosion to occur. Since the stainless steel shaft is more noble (-0.05V) than the silicone bronze propeller (-0.28V), the shaft will “steal” electrons from the propeller, and the propeller will begin to corrode. This corrosion often occurs first on the blades of the propeller, and the bronze will begin to have a pinkish hue.
Aluminum hull boats and Copper Anti-fouling paint
Another harmful galvanic couple that is often found in the marine environment is when an aluminum-hull boat. Aluminum (somewhere around -0.9V) and copper (-0.35V) make a couple with -0.26V of potential. The copper antifouling will “steal” the electrons from an aluminum hull, and cause the hull to corrode. Because of this galvanic corrosion potential, it’s important to paint aluminum boats with a copper-free antifouling paint. Don’t forget this for aluminum RIB dinghies! I’ve made this mistake myself, but because I painted the bottom of my brand new RIB with an epoxy barrier coat, I was OK. On the right, there’s a couple affiliate links to quality, aluminum-specific ablatives and barrier coats. Check them out for your aluminum RIB or any other aluminum-hulled boat.
https://www.westmarine.com/pettit-paint-eco-hrt-copper-free-antifouling-paint-P019371939.html
Galvanic Corrosion can happen anywhere on a boat
In Part 1 - Cathodic Protection, we established that that in order for galvanic corrosion to occur, we need four conditions to apply at the same time. In the saltwater marine environment, these conditions can happen to metals that are above the waterline, too. Salt spray and the salty, humid air that surrounds an ocean-going sailboat can serve as electrolytes; a weaker electrolyte than full on submersion in salt water, but still an electrolyte. For this reason, any time you’re working with metals above- and below-decks, it’s important to keep galvanic corrosion in mind.
To the left, I’ve put an affiliate link to Tef-Gel, a great product that lubricates moving parts and protects differing metals from galvanic corrosion. The Tef-Gel breaks the electron path between the two metals (serving as an electrical insulator). Without electrical connection between the two metals, galvanic corrosion can’t occur. Any time you can break one of the four necessary conditions needed for a galvanic couple, galvanic corrosion can’t occur.
Common Places to look for galvanic corrosion on a sailboat
Besides the obvious place, your underwater metals, galvanic corrosion is commonly found in connections to mast fittings (stainless screws in aluminum masts), aluminum rudder tillers (our Hunter charter boat has an aluminum tiller gripping a composite mast post, secured by stainless hardware and stainless steel pin - prone to galvanic corrosion), thru-hulls and other salt water components that unwisely mix different metals, and engine blocks. Many sailboat inboard engines contain sacrificial “pencil anodes” that should be checked periodically (every season). Aluminum/stainless steel mast fastener issues can sneak up, since they generally occur high-up in the mast in areas that are more difficult for inspection.
Conclusion
Tef-Gel is a great way to isolate fasteners exposed to open, salty air, but won’t solve all galvanic corrosion issues. If you suspect galvanic corrosion issues on your boat consider performing more tests (we’ll go over AgCl reference electrode testing in another article) or contact an ABYC-certified corrosion technician. You can search for a marine corrosion tech, and other ABYC-certified techs using ABYC’s tool here: https://abycinc.org/page/CTD.